How to Determine if a Solution Is Saturated
If you know what the. A Saturated Solution can be produced if you give sufficient sugar to your coffee or tea.
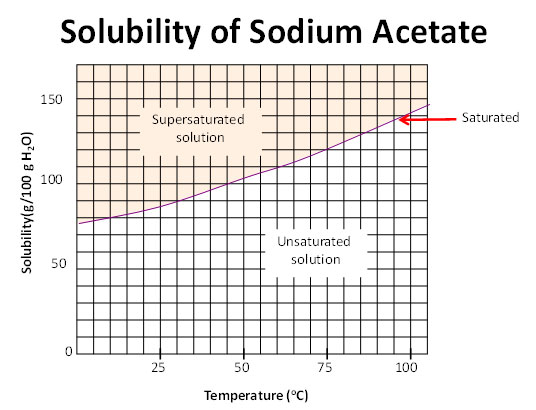
Types Of Solutions Saturated Supersaturated Or Unsaturated Texas Gateway
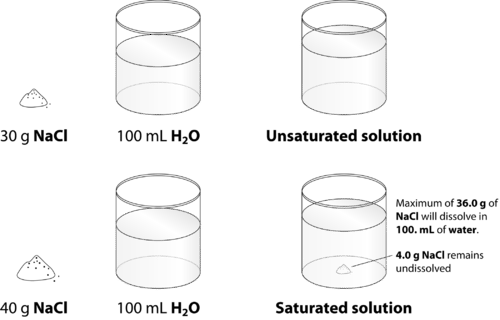
If the additional solute dissolves the solution is not saturated.
. Hot tea or coffee. Figure below illustrates the above process and shows the. A unsaturated solution has the tendency to dissolve more of the solute in it because of which the crystal or solute will reduces in size.
Correct option is A When a sample is taken out and cooled it forms small crystals rapidly. At a given temperature a solution can only hold a certain amount of soluteA saturated solution is. Weigh the empty saucer and record the weight.
If the solution is saturated a precipitate will form. A supersaturated solution is one that has more solute than it can hold at a certain temperature. K_spPb2Cl-2589 times 10-5 If a saturated solution contains an unknown.
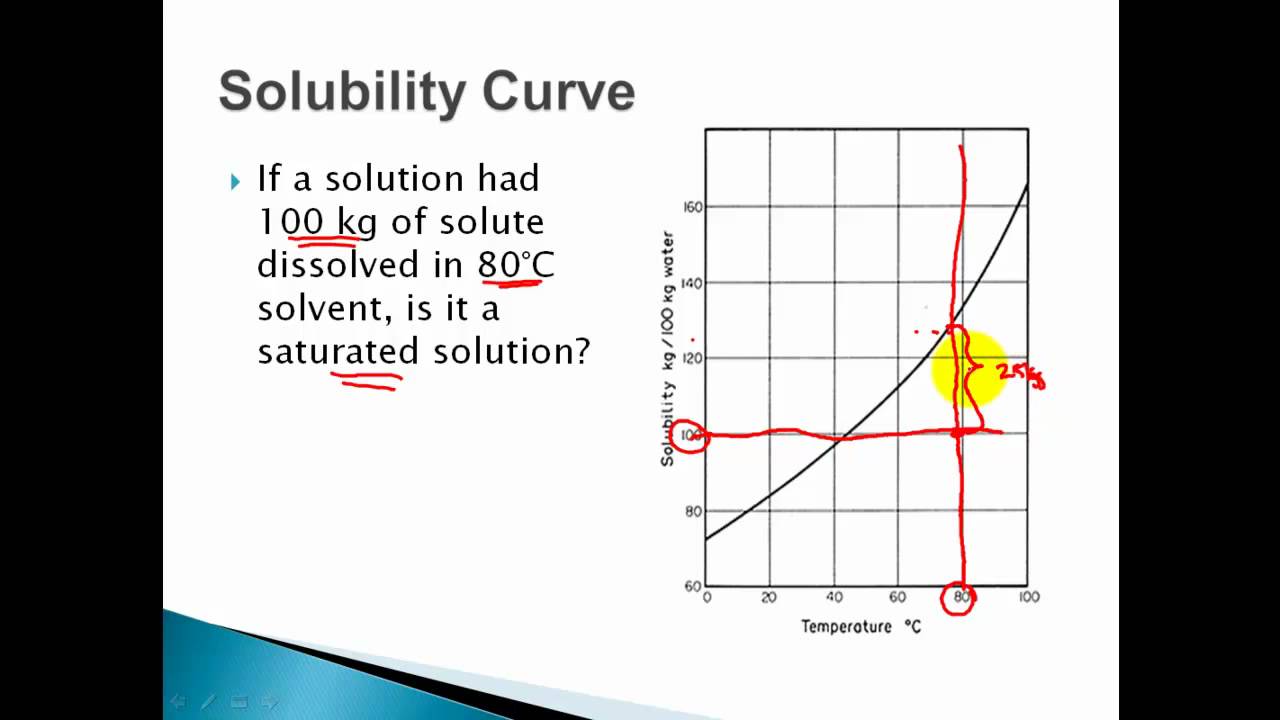
I recommend watching this in x125 - 15 speed In this video we go over how to determine the level of saturation in a solution unsaturated saturated or s. If we now heat the mixture to 50 C the remaining 9. Nine grams of solid remain on the bottom.
You shall know more or less when your solution is saturated. If we add 100 g of glucose to 100 mL water at 25 C 91 g dissolve. Add solute to a liquid until no more dissolves.
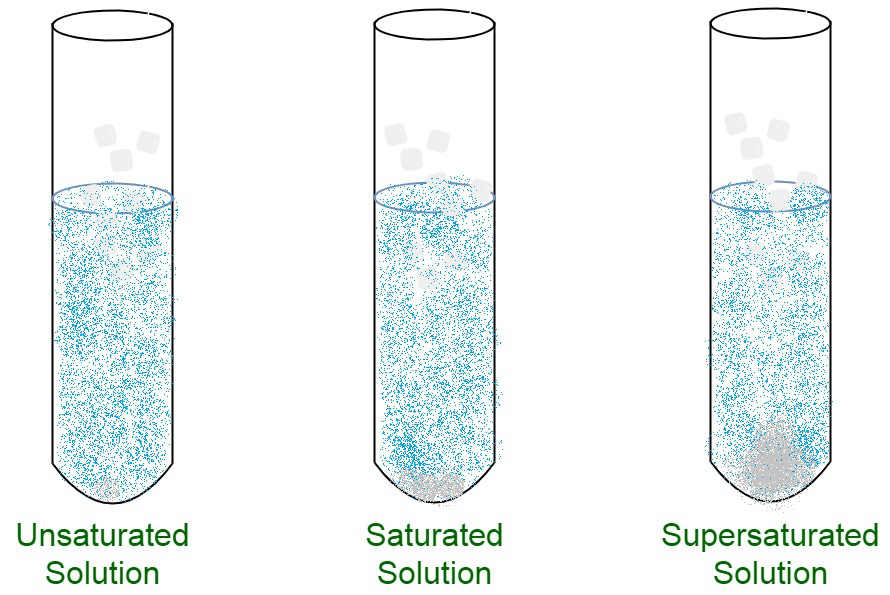
An unsaturated solution is a solution that contains less than the maximum amount of solute that is capable of being dissolved. We have a saturated solution. In chemistry after studying solutions and properties of the solution one can understand that a solution can reach a status of saturation.
The Ksp of silver phosphate would be which of the following. I determine how much solute will dissolve in the solvent at a given temperature. Youll know when the sugar starts dissolving youve reached the saturation point.
Label the underside of each saucer with tape one for each solution. Pour in 10-15 mL of the appropriate. Remove a sample and drop a bit of the solute into it.
Solubility curves can be used to. Evaporate solvent from a solution until it becomes saturated. Ii compare the solubilities of different solutes in the same solvent.
This is because unsaturated solutions have the weaker. A saturated solution is one in which the quantity of dissolved solute equals the saturation point of the solvent. This state is when the solution has reached a point in.
Typically when the temperature of a solution is increased more particles can be dissolved. A solvent can dissolve some particular types of solutes in it. Once the solution starts to crystallize or precipitate the solution is.
Electrical conductivity or impedance. If it does not the solution is saturated. Remember that only the dissolved aqueous species contribute to the equation.
In a saturated solution of silver phosphate the concentration of silver ion is 45 x 10-4 molL. Most solutes are less soluble as the temperature decreases. Alternatively let a small amount of solvent evaporate.
68 10-8 10 10-11 14 10-14 none of. Now if you want a more precise limit you need to measure with some equipment.

Solubility Curves Saturated Unsaturated Supersaturated Solutions Youtube

No comments for "How to Determine if a Solution Is Saturated"
Post a Comment